Institute for Systems Genetics
We take a systems approach to research in human biology and medicine.

The Institute for Systems Genetics at NYU Langone Health combines research in systems biology, genome engineering, human genetics, and computation. We take a systems approach to the wealth of information available in human biology and medicine.
Video: Building Big DNA in Yeast
The video below shows the steps involved in analyzing yeast colonies used to assemble chromosomes in the Genome Foundry at NYU Langone.
VIDEO: Jef Boeke, PhD, founding director of the Institute for Systems Genetics, describes the process of analyzing yeast colonies used to assemble chromosomes at NYU Langone’s Genome Foundry.
Our Mission

Our Facilities and Resources

Available Positions

Related News

A Synthetic Gene Helps Explain Transcription Across Species
Read Story

Genetic Change May Explain Why Humans Don’t Have Tails
Read Story

New Tool Reveals Gene Behavior in Bacteria
Read Story

Researchers Assemble Nine Synthetic Yeast Chromosomes
Read Story
Mice Survive COVID as Humans Do
Read Story
New AI Tool Makes Speedy Gene Editing Possible
Read Story
Cancer Cell Biology Predicts Response to Immunotherapy
Read Story
COVID-19 Infection Can Disrupt Gut Bacteria Diversity
Read Story
Dense Liquid Droplets Act as Cellular Computers
Read Story

Gene Changes May Impact Sudden Unexplained Death in Children
Read Story
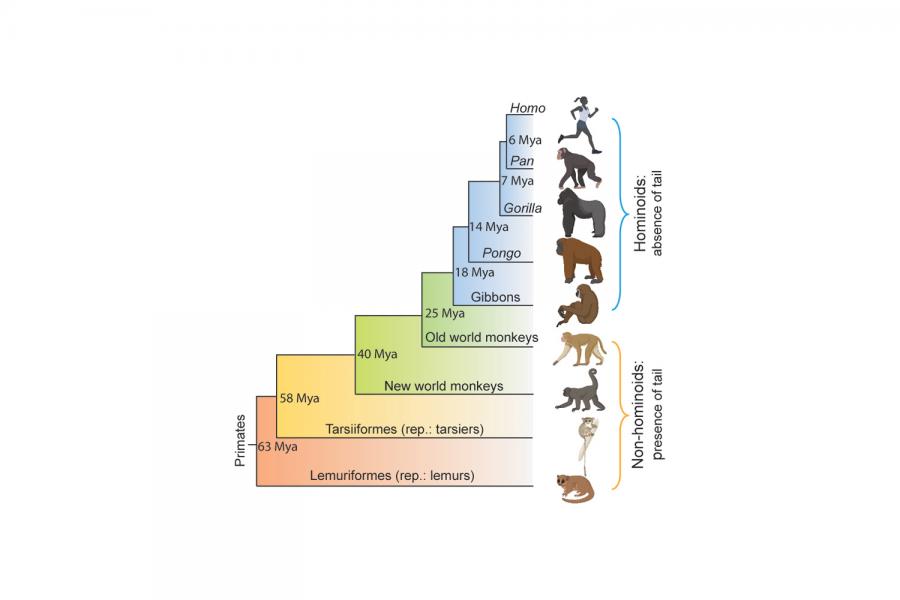
Genetic Study Suggests Why Humans No Longer Have Tails
Read Story

Investigating the Role of Genes in Osteoarthritis Therapy
Read Story
